This blog post is provided by Jinlin Chen and Chris Terry and tells the #StoryBehindThePaper for the paper ‘Natural enemies have inconsistent impacts on the coexistence of competing species’, which was recently published in the Journal of Animal Ecology.
A forgotten fruit in the kitchen will pretty quickly attract your attention by recruiting a swarm of fruit flies lingering around. Similar sights can be seen in the rainforest of Australian tropical mountains, where many Drosophila fruit fly species co-occur, apparently sharing the food resource. How might these species be able to coexist? To further thicken the ecological plot, there is rapid turnover in species composition across relatively small temperature gradients on these mountains1 and some species have been shown to be able to reproduce when alone outside of their observed range2. Understanding the complex patterns of co-occurrence on these mountains presents an intriguing test case for our understanding of ecological processes.
One classic answer to problems of community structure is the driving action of natural enemies – for example Paine’s seminal study3, where a starfish limits a dominant competitor (a mussel) allowing inferior competitors to co-exist. Might something similar be going on in our system?
Natural enemies of the flies certainly have a significant impact – a large proportion of fly pupae (10%-40%) in our samples are killed by parasitoid wasps that develop inside the larvae and pupae. At some times of the year, up to 100% of flies could be parasitized in samples from other studies.

Of course, it is not necessarily the case that natural enemies are an aid for coexistence. As generalist natural enemies may reduce the abundance of all competitors, it may be tempting to conclude that coexistence will be promoted through reduced competition for resources. However, there are a few dangerous gaps in such a chain of reasoning. For example, natural enemies may be more likely to drive the small population of the already inferior competitor to zero, thus reducing the chance of coexistence. Focusing on either the stabilizing or destabilizing aspects of the dynamics will both lead to unreliable conclusions. As a solution, modern coexistence theory allows the explicit framing of the question regarding pairwise species coexistence as a balance between niche differences promoting stabilisation and fitness differences fostering exclusion.
Paine’s experimental field approach of directly manipulating the numbers of starfish by flinging them from the rocks into the sea was not really translatable to our system. What we could do instead, taking advantage of the experimental amenability of Drosophila, was set up large laboratory trials to parameterise competition models, and this work is now published in Journal of Animal Ecology. We could then apply modern coexistence theory to ask with some precision how exactly parasitoids affected the ability for pairs of Drosophila species to coexist with each other.

The figure below shows an example of the results we found. Here without parasitism, it is likely that a more fecund competitor, D. sulfurigaster can exclude D. birchii (top left, in blue). However, when the competition trials are run in the presence of the parasitoid (middle, in red), the fitness difference between the pairs reduced as D. birchii was less susceptible to parasitism, and coexistence is more probable.
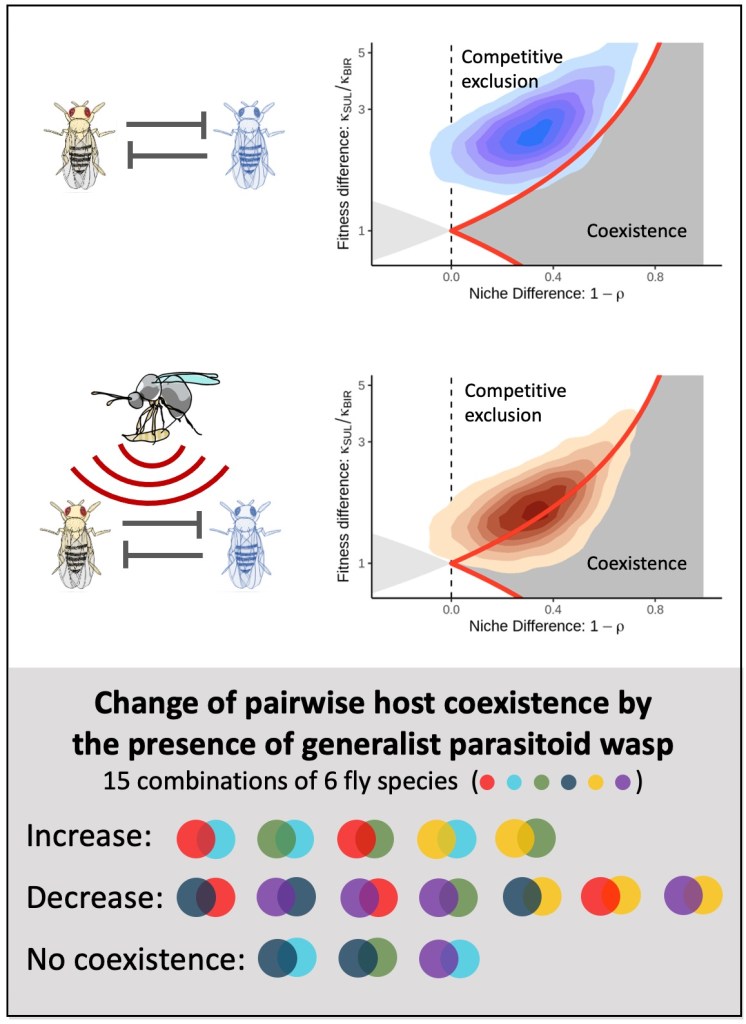
But one pair isn’t all that much help in determining what is going on at the community level. In all, we extended our analysis to all 15 pairwise combinations of six representative fly species from our Australian system. This was quite an undertaking, with two of us identifying and counting over 75000 flies from nearly 2000 tubes.
Across the set, rather than the neat pattern we described above, we saw a real mixed bag in how the parasitoids affected likelihood of coexistence: increases, decreases and cases of no change at all. Essentially, the less-susceptible fly species were not necessarily the inferior competitors. In a blow to hopes that there would be a way to shortcut the extensive competition trials, competitive ability could not be discerned just from the intrinsic fecundity – it was necessary to simultaneously take both their fecundity and intra- and inter-competitive coefficients into account.
In short, we cannot expect generalist predators to be consistent drivers of coexistence. In accordance with other empirical studies, predominantly in plant systems, the regulation of co-existence by the other trophic levels appears highly variable4,5,6. Nevertheless, our and others’ studies have demonstrated that we have the theoretical framework and analytical tools to study the seemingly messy effects in various biological systems.
The Australian fruit fly-parasitoid system may not be the most glamorous, but it does enable the study of ’realistically complicated’ competitive and trophic networks in both laboratory conditions and the field. Our laboratory system includes all ten of the most prevalent fruit fly species along an altitude gradient and several of their native parasitoid wasps, each with a distinctive natural history. This system can offer a uniquely powerful test for the natural enemy-mediated coexistence among insects whose interactions are naturally assembled.
Looking to the future, we are hopeful that we can upscale the pairwise relationships to multi-species interactions in a well-controlled manner, and extend to more trophic interactions by addition of parasitoid and hyper-parasitoid species. Although there is some distance to go, we are optimistic that we are on track to empirically understand how diversity is regulated by simultaneous interactions with different trophic levels.
Citations:
- Terry, J., Chen, J., & Lewis, O. (2021). Natural enemies have inconsistent impacts on the coexistence of competing species. Journal of Animal Ecology.
- O’Brien, E. K., Higgie, M., Reynolds, A., Hoffmann, A. A., & Bridle, J. R. (2017). Testing for local adaptation and evolutionary potential along altitudinal gradients in rainforest Drosophila: beyond laboratory estimates. Global change biology, 23(5), 1847-1860.
- Paine, Robert T. “Food web complexity and species diversity.” The American Naturalist 100.910 (1966): 65-75.
- Petry, W. K., Kandlikar, G. S., Kraft, N. J., Godoy, O., & Levine, J. M. (2018). A competition–defence trade‐off both promotes and weakens coexistence in an annual plant community. Journal of Ecology, 106(5), 1806-1818.
- Kandlikar, G. S., Yan, X., Levine, J. M., & Kraft, N. J. (2021). Soil Microbes Generate Stronger Fitness Differences than Stabilization among California Annual Plants. The American Naturalist, 197(1), E000-E000.
- Mordecai, E. A. (2013). Despite spillover, a shared pathogen promotes native plant persistence in a cheatgrass‐invaded grassland. Ecology, 94(12), 2744-2753.
Read the paper
Read the full paper here: Terry, J.C.D., Chen, J. and Lewis, O.T. (2021), Natural enemies have inconsistent impacts on the coexistence of competing species. Journal of Animal Ecology. Accepted Author Manuscript. https://doi.org/10.1111/1365-2656.13534

One thought on “Can a generalist parasitoid act like Paine’s starfish?”